Exploring the Modalities of Teeth Whitening
Tooth whitening has become one of the most sought-after cosmetic dental procedures, offering individuals a brighter smile and enhanced confidence. This popular and conservative method removes dental stains and discolors teeth through chemical processes. The primary mechanism involves the oxidation of chromogenic materials by free radicals released from peroxide compounds. While the process is widely effective, various modalities of teeth whitening cater to diverse patient needs and circumstances.
In-Office Teeth Whitening (Power Bleaching)
Power bleaching, also known as in-office whitening, is among the most effective and rapid methods of teeth whitening. This procedure employs high concentrations of hydrogen peroxide (HP) or carbamide peroxide (CP), with the most common concentration of hydrogen peroxide ranging from 30% to 35%. These potent agents penetrate the enamel to break down stains and discoloration efficiently.
Mechanism of Action
The whitening effect of power bleaching is achieved through oxidation. When the peroxide compound breaks down, it releases free radicals that react with the chromogenic materials responsible for tooth discoloration. This reaction disrupts the double bonds in these molecules, lightening the color of the teeth.
Advantages
Speed: In-office whitening typically produces noticeable results in a single session, making it ideal for patients seeking immediate improvements.
Professional Supervision: The procedure is performed under the care of a dentist, ensuring precision and safety.
Customized Approach: Dentists tailor the treatment to address the specific needs of each patient, optimizing outcomes.
Safety Considerations
Power bleaching’s high peroxide concentrations can pose risks to oral soft tissues. Accidental contact with gums, lips, or other soft tissues can cause temporary caustic injuries. However, these injuries usually heal within one to two weeks without complications. To mitigate risks, dentists use protective measures, such as rubber dams or specialized gels, to shield the soft tissues.
Recent studies have raised concerns about the use of intense light sources during in-office whitening. These lights, which were once believed to enhance the bleaching effect, can elevate dental pulp temperatures, potentially leading to tooth sensitivity or pulpal damage. Researchers now advise caution when using light-activated systems, particularly with high concentrations of hydrogen peroxide. Many experts suggest avoiding light activation altogether, as it may not significantly improve the whitening effect.
He LB et al. The effects of light on bleaching and tooth sensitivity during in-office vital bleaching: a systematic review and meta-analysis. J Dent 2012; 40(8):644-53.
At-Home Whitening Kits
At-home whitening kits offer a convenient and cost-effective alternative to in-office treatments. These kits typically contain lower concentrations of hydrogen peroxide or carbamide peroxide, ranging from 10% to 20%.
Mechanism of Action
Similar to power bleaching, at-home kits rely on peroxide compounds to release free radicals that oxidize chromogenic materials. However, the lower concentration of active agents means the process is slower, requiring repeated applications over several days or weeks.
Advantages
Affordability: At-home kits are generally less expensive than professional in-office treatments.
Convenience: Patients can use these kits at their own pace, in the comfort of their homes.
Milder Formulation: Lower peroxide concentrations reduce the risk of sensitivity and tissue irritation.
Limitations
Consistency: Achieving uniform results can be challenging without professional supervision.
Extended Duration: At-home whitening requires more time to achieve noticeable results compared to in-office treatments.
Potential Misuse: Improper application or overuse can lead to uneven whitening or irritation.
It should be noted that successive applications of even just 10% carbamide peroxide, a supposedly mild formulation, has shown to promote severe cytotoxic effects in the dental pulp.
Lima AF.(link is external) Toxic effects of daily applications of 10% carbamide peroxide on odontoblast-like MDPC-23 cells. Acta Odontol Scand. 2013 Sep; 71(5): 1319-1325.
Soares DG. Effect of fluoride-treated enamel on indirect cytotoxicity of a 16% carbamide peroxide bleaching gel to pulp cells. Braz Dent J. 2013;24(2):121-7.
Soares DG. Transenamel and transdentinal cytotoxicity of carbamide peroxide bleaching gels on odontoblast-like MDPC-23 cells. Int Endod J. 2011 Feb;44(2):116-25.
Another study demonstrated direct cytotoxic effects on odontoblast-like cells.
Dias Ribeiro AP.(link is external) Cytotoxic effects of a 35% hydrogen peroxide bleaching gel on odontoblast-like MDPC-23 cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009; 108(3): 458-64.
Over-the-Counter Whitening Products
Over-the-counter (OTC) whitening products, such as whitening strips, toothpaste, and gels, are widely available and easy to use. These products cater to individuals seeking mild to moderate whitening effects without the need for professional intervention.
Mechanism of Action
OTC products generally contain lower concentrations of hydrogen peroxide or alternative whitening agents like baking soda. The active ingredients target surface stains and may provide gradual whitening with regular use.
Advantages
Accessibility: OTC products are readily available at pharmacies and online stores.
Affordability: They are among the most cost-effective whitening options.
Ease of Use: Most OTC products are straightforward and require minimal preparation.
Limitations
Limited Effectiveness: OTC products are less potent than professional treatments, making them less effective for severe discoloration.
Prolonged Usage: Achieving noticeable results can take weeks or months of consistent use.
Safety Concerns: Frequent use of abrasive whitening toothpaste may erode enamel over time, leading to increased sensitivity.
Some patients may have used consumer-available bleaching products, such as paint-on bleaching solutions. These products were shown to adversely affect microhardness compared to controls and regimens with 10% of carbamide peroxide.
One study suggested that consumers should be made aware of this effect on enamel:
Leonard RH. Effect on enamel microhardness of two consumer-available bleaching solutions when compared with a dentist-prescribed, home-applied bleaching solution and a control. J Esthet Restor Dent 2005; 17(6): 343-50.
Several studies described a negative effect of bleaching agents on hardness and the surface morphology of enamel:
Lopes GC et al. Effect of bleaching agents on the hardness and morphology of enamel. J Esthet Restor Dent 2002; 14(1): 24-30.
Grobler SR et al. Effect of various tooth-whitening products on enamel microhardness. SADJ 2009; 64(10): 474-479.
Majeed A et al. Effect of four different opalescence tooth-whitening products on enamel microhardness. SADJ 2008; 63(5): 282-284, 286.
Mishima FD. The effect of bleaching on the enamel surface and the tensile force to debond orthodontic brackets. J Orthodont 2009; 36(4): 236-242.
Bodanezi A.(link is external) Surface Modifications on Aesthetically Restored Teeth following Home Bleaching with 16% Peroxide Carbamide. Eur J Dent. 2011; 5(2): 157–162.
Some authors found increased surface roughness and a higher retention of Streptococcus Mutans (the bacterium that is the main contributor to tooth decay):
Hosoya N. Changes in enamel surface roughness and adhesion of Streptococcus mutans to enamel after vital bleaching. Journal of Dentistry 2003; 31(8): 543–548.
Natural Whitening Remedies
Natural remedies for teeth whitening have gained popularity among individuals seeking chemical-free alternatives. Common methods include using baking soda, activated charcoal, or oil pulling with coconut oil.
Mechanism of Action
Baking Soda: Acts as a mild abrasive, helping to remove surface stains.
Activated Charcoal: Absorbs impurities and surface stains through its porous structure.
Oil Pulling: Involves swishing oil in the mouth to remove bacteria and plaque, contributing to a cleaner appearance.
Advantages
Affordability: Most natural remedies are inexpensive and easily accessible.
Safety: They typically lack harsh chemicals, reducing the risk of irritation.
Holistic Appeal: Many individuals prefer natural methods for their perceived health benefits.
Limitations
Limited Evidence: Scientific support for the efficacy of natural whitening remedies is sparse.
Slow Results: These methods are generally less effective than peroxide-based treatments.
Potential Risks: Overuse of abrasives like baking soda or activated charcoal can damage enamel.
Safety and Effectiveness Across Modalities
When considering teeth whitening options, safety and effectiveness are paramount. Factors such as the concentration of active agents, duration of application, and individual sensitivity levels play critical roles in determining outcomes. Consulting a dentist before initiating any whitening treatment ensures that the chosen method aligns with the patient’s oral health and aesthetic goals.
Managing Sensitivity
Tooth sensitivity is a common side effect of whitening procedures, particularly those involving peroxide compounds. Patients can minimize sensitivity by:
Using desensitizing toothpaste before and after whitening.
Limiting the frequency and duration of treatments.
Opting for lower concentrations of active agents.
Maintenance of Results
Maintaining whitening results requires consistent oral hygiene practices and lifestyle adjustments. Avoiding stain-causing substances, such as coffee, tea, red wine, and tobacco, can prolong the effects of whitening treatments. Regular dental cleanings also help prevent the accumulation of new stains.
Conclusion
Teeth whitening encompasses a variety of modalities, each with unique benefits and limitations. From rapid in-office treatments to gradual at-home methods and natural remedies, individuals have numerous options to achieve a brighter smile. Professional supervision remains critical for ensuring safety and optimizing results, particularly for those undergoing high-concentration treatments. By understanding the mechanisms, advantages, and considerations of each modality, patients can make informed decisions that align with their needs and preferences, paving the way for a confident and radiant smile.
Additional Information
Peroxide bleaching agents do not damage surfaces of existing composite fillings. While they are not bleached as teeth, some composite materials show some color alteration after bleaching procedures. In addition, the bond strength of restorative material to dentin and the flexural strength of dentin have been shown to be reduced after the use of higher concentrations of bleaching agents.
Mourouzis P et al. Effect of in-office bleaching agents on physical properties of dental composite resins. Quintessence Int 2013; 44(4): 295-302.
Vieira C et al. Effect of high-concentrated bleaching agents on the bond strength at dentin/resin interface and flexural strength of dentin. Braz Dent J 2012; 23(1): 28-35.
Shinohara MS et al. Shear bond strength evaluation of composite resin on enamel and dentin after nonvital bleaching. J Esthet Restor Dent 2005; 17(1): 22-9; discussion 29.
In severe cases of dental staining, such as tetracycline stains of teeth, even a combination of power bleaching and professional teeth bleaching at home may not yield desirable results. In such cases, the only remedy to create a natural beautiful look is the placement of porcelain veneers.

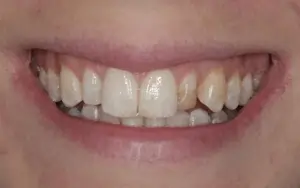
This patient did not like the underdeveloped right and the underdeveloped and discolored left lateral incisor as well as the associated visual dominance of the left upper canine. She also has signs of dental fluorosis, called dental mottling, which is caused by a developmental disturbance of dental enamel due to excessive exposure to fluoride during tooth development. Dental fluorosis is commonly treated by dental bleaching and microabrasion to reduce superficial staining, and composite restorations (bonding) and porcelain veneers for more significant aesthetic challenges, such as in this case.
She completed a professional tooth bleaching at home. In addition, she received porcelain veneers at her upper lateral incisors and some minor reshaping of the disto-incisal line angle at the right upper central incisor (ameloplasty). The tooth whitening did not eliminate the white mottling on most tooth surfaces, but it removed the stains that were visible on some teeth. This conservative approach created a fresher look and a more harmonious smile, even though the degree of the achieved tooth whitening is subtle.

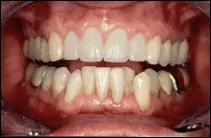
This patient had misaligned and discolored upper and lower teeth. He decided to straighten and lighten his upper teeth with multiple veneers and to just bleach his lower teeth with a professional home-bleaching regimen.
After completion of his treatment, patient had generally whiter teeth and a more balanced and harmonic upper tooth line.
Since it is usually the dentin, underlying the relatively translucent and colorless dental enamel, that gives a tooth it's chroma, whitening agents need to penetrate the deeper surfaces of the tooth structure. Dental enamel is permeable due to the inter-prismatic spaces of its crystalline configuration. While it is not exactly known how peroxide leads to color change, it is widely assumed that a chemical reaction of hydrogen peroxide and organic chromophores (Marcovic I. et al, 2010). This so called Dominant Chromophore Effect Theory assumes that hydrogen peroxide is attracted to areas with high electron density, which is a property of chromatic molecules. Structural change of these molecules makes them "whiter," which in turn lightens the tooth.
Marcovich I et al. Effects of bleaching agents on human enamel light reflectance. Operative Dentistry 2000; 35(4): 405-411.
As stated above, the amount of penetrations of the bleaching agent into the tooth correlates with the amount of tooth whitening. The extent of penetration is depends on
- exposure time (Hanks CT et al, 1993)
- surface area
- concentration of the hydrogen hydroxide (Bowles WH et al, 1987; Joiner A, 2006)
- size of dentinal tubule openings (Camps J et al, 2007)
- location at tooth
- acid etching of tooth surface (Benetti AR et al, 2004; Patri G et al, 2013)
- light activation (Camargo SE et al, 2009)
Hanks CT et al. Cytotoxicity and dentin permeability of carbamide peroxide and hydrogen peroxide vital bleaching materials, in vitro. J Dental Res 1993; 72(5): 931-938.
Bowles WH et al. Pulp chamber penetration by hydrogen peroxide following vital bleaching procedures. J Endod 1987; 13(8): 375-377.
Joiner A. The bleaching of teeth: a review of the literature. J Dentistry 2006; 43(7): 412-419.
Camps J et al. Time-course diffusion of hydrogen peroxide through human dentin: clinical significance for young tooth internal bleaching. J Endod 2007; 33(4): 455-459.
Benetti AR et al. In vitro penetration of bleaching agents into the pulp chamber. Int Endod J 2004; 37(2): 120-124.
Patri G et el. An in vitro spectrophotometric analysis of the penetration of bleaching agent into the pulp chamber of intact and restored teeth. J Clin Diagn Res; 7(12): 3057-3059.
Camargo SE et al. Penetration of 35% hydrogen peroxide into the pulp chamber in bovine teeth after LED or Nd:YAG laser activation. Eur J Esthetic Dentistry; 4(1): 82-88.
Leading Cosmetic Dentist San Francisco, Dr. Jorg-Peter Rabanus is accredited by the American Academy of Cosmetic Dentistry.



