Hybrid Layers: The Fusion of Science and Dentistry
The development of the chemical elements of dentin bonding has led to a dental revolution. The spectrum of dental treatment modalities, such as porcelain veneers, has significantly widened and become more predictable and safer today.
Recently, there has been a discussion about "touch-cure polymerization" at the interface between the dentin surface of the prepared tooth and the dental resin/composite. The idea is that the polymerization starts at the tooth surface, hence pulling the dental composite towards the dentin and rendering a more intimate and stronger bond of the porcelain veneer. Research is ongoing and has been controversial:
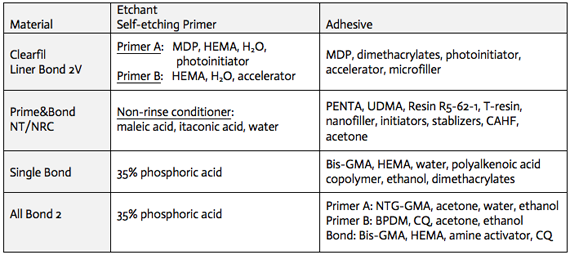
Examples of dental adhesive systems:
Animated bonding sequence
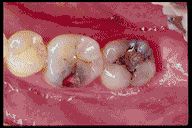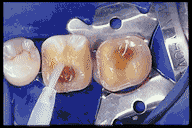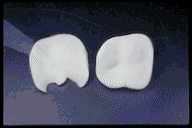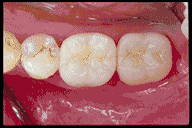
Anatomy of the Tooth and Its Role in Bonding
A tooth’s visible structure is composed of two principal hard tissues:
Dental Enamel: The outermost protective layer, composed primarily of densely packed hydroxyapatite crystals. Enamel’s crystalline nature enables micro-retention through acid etching.
Dentin: The inner layer, forming the bulk of the tooth. Unlike enamel, dentin contains a higher proportion of organic material, such as collagen, along with water and hydroxyapatite. This layer surrounds the dental pulp, which houses nerves and blood vessels.
Enamel bonding was initially a prerequisite for dental restorations due to its stable structure and low water content. However, advancements in bonding agents have made dentin bonding a viable option, expanding treatment possibilities.
The Transition from Enamel to Dentin Bonding
Enamel Bonding
Traditional bonding techniques relied heavily on enamel due to its highly mineralized and hydrophobic structure. Acid etching created microscopic retentive patterns in enamel, enabling secure adhesion of hydrophobic dental resins. The irregular surface produced by this etching allowed for strong mechanical micro-retention.
Challenges with Dentin
Dentin posed significant challenges for bonding due to its high water content and organic matrix. Early resins were ineffective on dentin as they were hydrophobic and failed to adhere to the wet, collagen-rich surface. Overcoming these limitations required innovative bonding agents capable of interacting with both hydrophilic and hydrophobic components.
The Hybrid Layer: A Breakthrough in Bonding Technology
The development of the hybrid layer revolutionized dentin bonding by creating a robust interface between the tooth and restorative material. The process involves:
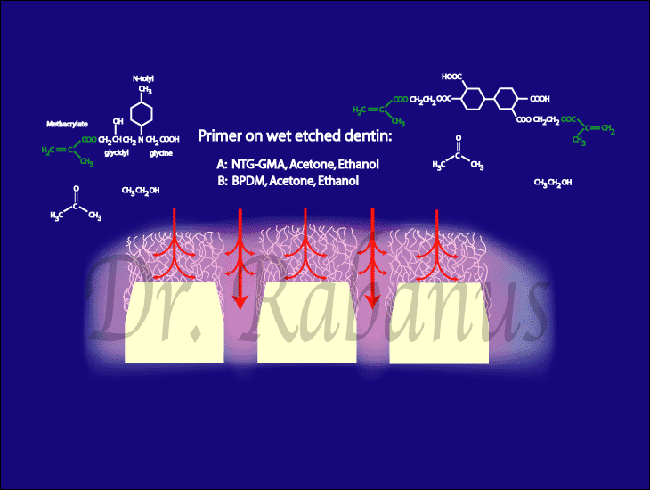
Acid Etching: Dissolves the mineralized surface of dentin to expose the collagen network. This step requires careful moisture control to prevent the collapse of collagen fibers.
Primer Application: Hydrophilic resins are introduced, diffusing into the collagen matrix and forming a stable hybrid layer.
Resin Infiltration: Synthetic polymers lock into the acid-etched dentin, creating a strong chemical and physical bond.
This hybridized dentin layer is a true fusion of natural and synthetic materials. It provides:
Sealing: Prevents leakage and bacterial infiltration.
Strength: Enhances the durability of restorations.
Resistance: Offers protection against future acid attacks.
Technical Considerations in Bonding
Maintaining a Clean Environment
A clean, uncontaminated tooth surface is essential for successful bonding. The use of rubber dams ensures that saliva and other biological molecules do not interfere with the process. Additionally, maintaining the proper moisture balance during etching and primer application is crucial to preserving the collagen network.
Polymerization Techniques
Recent advancements have introduced concepts such as touch-cure polymerization, which initiates curing at the dentin surface. This technique aims to:
Improve Bond Strength: By pulling the composite material towards the tooth surface.
Enhance Intimacy: Creates a more cohesive bond between the restorative material and dentin. While promising, this method remains a subject of ongoing research and debate.
Applications and Benefits of Bonding
The advancements in bonding technology have broadened the scope of dental treatments, including:
Restorative Dentistry
Fillings: Composite resins bonded to tooth surfaces provide natural-looking, durable restorations.
Crowns and Veneers: Bonded porcelain restorations require minimal tooth reduction, preserving natural structure.
Fracture Resistance: Bonded teeth are more resistant to breaking than untreated ones.
Cosmetic Dentistry
Smile Design: Bonding techniques enable precise reshaping and color correction of teeth.
Repairing Damage: Chipped, cracked, or worn teeth can be seamlessly restored.
The Impact of Bonding on Modern Dentistry
The integration of bonding agents has led to:
Minimized Tooth Reduction: Enabling conservative treatments that prioritize natural structure.
Improved Aesthetics: Bonded restorations blend harmoniously with natural teeth.
Enhanced Longevity of Porcelain Veneers: Properly bonded restorations exhibit increased durability and resistance to wear.
Future Directions in Bonding
Research continues to refine bonding techniques, focusing on:
Improved Materials: Developing agents with higher adhesion strength and versatility.
Advanced Polymerization: Exploring new curing methods for stronger bonds.
Biocompatibility: Enhancing the integration of synthetic materials with natural tooth structures.
Conclusion
Tooth bonding exemplifies the synergy of science and artistry in dentistry. From its origins in enamel bonding to the revolutionary hybrid layer, the technology has redefined how dentists approach restorations and cosmetic enhancements. As advancements continue, the future of tooth bonding promises even greater possibilities for creating durable, aesthetically pleasing, and minimally invasive dental solutions.
Please find Part 1 by clicking here and learn more about the basics of bonding as used in cosmetic dentistry and explained in the 'Procedures' section.
References:
DiMatteo AM. Esthetic Bonding: How to avoid catastrophe before it starts. Inside Dentistry. Jan. 2017; 34-38.
Sorensen JA et al. Shear bond strength of adhesives to dentin with varying moisture conditions. J Dental Res. 2016; Spec Iss. Ab#1342.



